Abstract
INTRODUCTION: Chronic myeloid leukemia (CML) is a bone marrow and blood disorder accounting for 15% of adult leukemia. A shift in CML management has occurred over the past decade with the introduction of tyrosine kinase inhibitors (TKIs), changing CML status from fatal to a chronic, lifelong illness. However, an association between TKI use and cardiovascular events has been observed. This study aimed to compare major adverse cardiac events (MACE), arterial occlusive events (AOEs), and venous thrombotic events (VTEs) among CML patients in chronic phase (CP-CML) treated with different TKIs.
METHODS: A retrospective observational study of adult (aged ≥18 years) CP-CML patients prescribed a TKI was conducted using the IBM® MarketScan® Research Databases from July 2012-June 2017. The index date was defined as the index drug prescription date, identified based on TKI use during the identification period (January 2013-December 2016) in hierarchical order: ponatinib, bosutinib without ponatinib, and other TKIs (imatinib, dasatinib, nilotinib) excluding ponatinib and bosutinib. Patients were required to have continuous health plan enrollment for ≥6 months pre-index date (baseline period) and at least 6 months post-index date (follow-up period). Patients with use of one or two previous TKI(s) before the index date were examined separately. Cardiovascular events occurring through the earliest of discontinuation of index TKI, switch to another TKI, or end of follow-up period using ICD-9/10-CM diagnosis codes were calculated as the number of events per 100 person-years. MACE was defined as a composite of stroke (hemorrhagic stroke and ischemic stroke), myocardial infarction, and inpatient death; AOEs included cardiovascular, cerebrovascular, and peripheral vascular events; VTEs included pulmonary embolism and deep vein thrombosis.
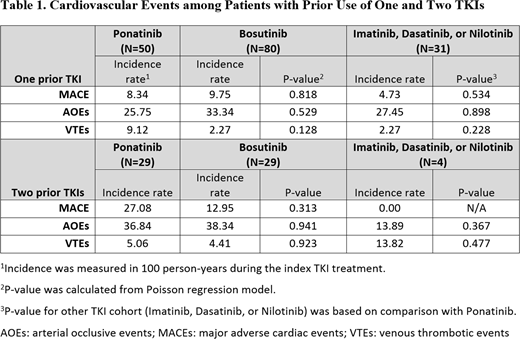
RESULTS: After applying the selection criteria, 161 patients had one previous use of a TKI, with 50 ponatinib, 80 bosutinib and 31 other TKI patients. Mean ages were 54, 57, and 58 years for ponatinib, bosutinib, and other TKI cohorts, respectively. Most ponatinib patients initiated treatment with a dose of 45 mg (60%); most bosutinib patients initiated with a dose of 500 mg (53%) . For patients with use of one previous TKI, the average Charlson Comorbidity Index score was 1.4 for ponatinib, 1.8 for bosutinib and 0.8 for other TKI patients. Common baseline comorbid conditions by drug included anemia (ponatinib: 50%; bosutinib 31%; other TKI: 19%), hypertension (ponatinib: 32%, bosutinib: 43%, other TKI: 29%), and diabetes (ponatinib: 16%; bosutinib: 28%; other TKI: 10%). CP-CML patients were observed to have cardiovascular events prior to index TKI use, especially MACE (ponatinib: 4%, bosutinib: 16%, other TKI: 3%), and AOEs (ponatinib: 12%; bosutinib: 25%; other TKI: 19%). During the follow-up period, no significant differences were found for cardiovascular events across patients with TKI use (Table 1); the incidence of MACE was 4.7-8.3, AOEs: 25.8-33.3, and VTE: 2.3-9.1 (in 100 person-years). For those with use of two types of TKIs before the index date, 29 ponatinib, 29 bosutinib, and 4 other TKI patients were identified, with an average age of 51, 59, and 65 years, respectively. A similar trend was observed for patients with use of two prior TKIs.
CONCLUSION: CP-CML patients treated with different TKIs (ponatinib, bosutinib, imatinib, dasatinib, and nilotinib) did not have different incidence of cardiovascular events (MACE, AOEs, VTEs) in this small cohort of real-world patients with ≥6-month of follow-up. The results were consistent among patients with prior use of one and two TKI types.
Levy:Takeda (Millennium Pharmaceuticals, Inc.): Consultancy. Xie:STATinMED Research: Employment. Wang:STATinMED Research: Employment. Neumann:Takeda (Millennium Pharmaceuticals, Inc.): Employment. Srivastava:Takeda (Millennium Pharmaceuticals, Inc.): Employment. Naranjo:Takeda (Millennium Pharmaceuticals, Inc.): Employment. Zhang:STATinMED Research: Employment. Dalal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd, Cambridge, MA, USA: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal